Now testing patients and controls in rural and regional NSW
We are now beginning in-person testing of Parkinson’s patients and controls in rural and regional NSW. We have already tested our first Tamworth cohort, and will be testing in Newcastle early next month. Ph.D. student Alycia Messing is heading up the study, and is particularly keen to recruit age-matched controls over 60 for the study. The study involves a non-invasive brain scan using EEG (electroencephalogram), some measures of balance (postural sway), a finger tapping task and some cognitive measures. For the Parkinson’s patients we are also taking some clinical measurements. We’ll be doing this across 18 months at 3 time points, and we’ll come to your town. If you’re keen to participate, please contact Alycia on amessin2@myune.edu.au
National survey recently completed
We have recently completed a national survey aimed at building a better understanding of how people with Parkinson’s and their families are coping with the disease. We considered it particularly important to have representation from people in rural and regional Australia. This was be the first Australia-wide survey specifically looking at quality of life for people with Parkinson’s in rural and regional Australia compared to those in metropolitan areas. Results will be released soon.
Report for UNE Media by Amanda Burdon
Researchers at UNE’s School of Psychology have recently secured funding from the Perpetual Impact Philanthropy Foundation to use mobile technology and machine learning to help solve the mystery of how to determine the progression of Parkinson’s disease. This study extends a previous study (also headed by Dr. Deborah Apthorp while she was at the Australian National University, and also funded by Perpetual), to extend its reach to rural and regional NSW. Crucially, the team will continue to collaborate with colleagues at the Australian National University, tapping the expertise of clinicians and data scientists at The Canberra Hospital and ANU’s Research School of Computer Science.
Currently, when someone is diagnosed with Parkinson’s disease it is difficult to determine what type of Parkinson’s they have or how quickly the condition will progress. This project aims to tracks a range of early symptoms to determine if any can be used as an indicator of progression.
“The issue with Parkinson’s disease is that some people can do well for quite a long time, while others within five or 10 years will be constrained to a nursing home,” Dr Apthorp said.
“There are different types of Parkinson’s that can look similar at the point of onset, but they progress very differently. We are hoping the information we collect will differentiate between these different conditions.
“Ultimately we’d like doctors and other primary health professionals to be able to conduct simple, accurate tests that can help predict how the disease is likely to progress.”
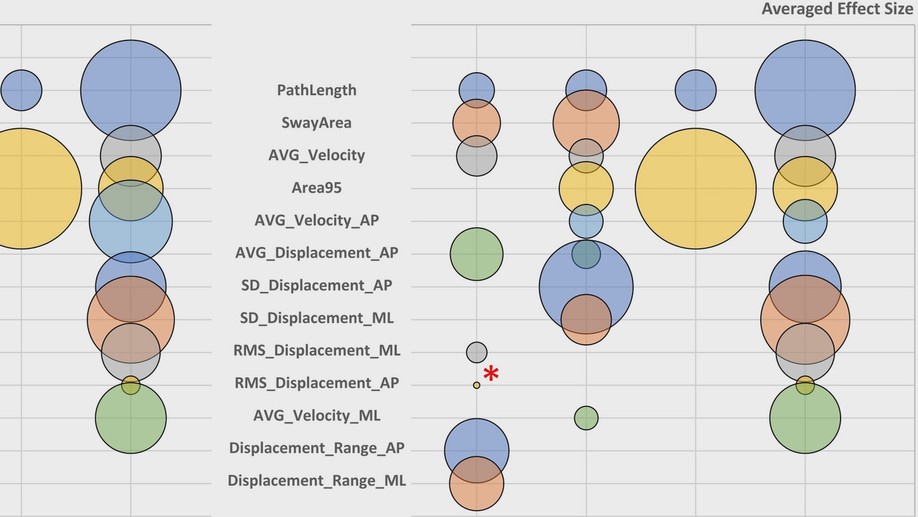
The research is using brain imaging EEG techniques, as well as eye tracking, visual perception, simple finger tapping tests, and postural sway, in addition to more traditional clinical measures.
“Human posture is an inherently unstable system, so you’re constantly making small corrections,” Dr Apthorp said. “When you get Parkinson’s disease it becomes harder and harder to maintain that upright posture, and you have to think more about it. Eventually, as the disease gets further along, you might start to fall or have difficulty walking.
“There is also some evidence that speed of eye movement is related to parts of the brain that are impacted by Parkinson’s, so we plan to look at all these measures together”
This funding will enable the researchers to equip a mobile research lab custom-built into a van, with EEG, eye tracking equipment, a force plate for measuring balance, and the option to add other technology as it becomes available. The van will be equipped with solar panels to power the instruments, and lined with light-limiting curtains to provide a consistent visual environment anywhere it can be parked.
Initially, the team aims to extend the work from work previously funded by Perpetual at ANU, extending research on machine learning in Parkinson’s disease across remote and rural areas of NSW and southern QLD, based at the University of New England. The overall goal is to develop technology for remote assessment of symptoms associated with neurological disorders like Parkinson’s disease. However, this facility will also be extended to research on Multiple Sclerosis and Diabetes via Dr. Apthorp’s links with ANU’s winning 2017 ANU Grand Challenge team, “Our Health In Our Hands”.
There is a stark lack of accessibility to specialised medical care in remote and rural regions of Australia. For instance, patients in the New England region do not have access to a specialist neurologist without travelling several hours. Since Parkinson’s patients struggle with mobility, they often rely on public transport, which is also limited in these regions. This project offers the possibility that inexpensive technologies such as balance plates (or even mobile phones), combined with other forms of non-invasive, simple data, could offer sufficient information for individuals to track the state of their health, disease progression and response to medication in the comfort of their own homes, or in a simple rural clinic setting. This team have advanced expertise in signal processing and data analysis to develop these new measures, and now we seek the ability to reach patients and empower them to participate in research that can shape their future.